hATTR-PN treatment options
Medications, clinical monitoring, and symptom management can all play a role in hereditary transthyretin amyloidosis with polyneuropathy (hATTR-PN) treatment.
Also known as familial amyloid polyneuropathy, hATTR-PN is a genetic condition marked by polyneuropathy, or damage to multiple peripheral nerves found outside the brain and spinal cord. It is an inherited form of transthyretin (TTR) amyloidosis, a group of disorders characterized by the accumulation of misfolded TTR proteins in different tissues and organs.
Several approved and experimental TTR amyloidosis treatment options can slow or stop disease progression in hATTR-PN, but none can reverse nerve damage. Early diagnosis and initiation of hATTR-PN treatment are crucial for preventing or delaying further damage.
Disease-modifying therapies
In recent years, several disease-modifying therapies targeting the underlying causes of hATTR-PN have been introduced to supplement existing management strategies for hATTR-PN. These therapies aim to prevent the accumulation of misfolded TTR protein deposits in peripheral nerves, which are the main drivers of nerve damage in hATTR-PN. Mutations in the TTR gene, which contains instructions for cells to produce TTR, destabilize the protein’s structure, making it prone to misfold and form toxic protein aggregates called amyloid deposits.
By addressing these processes, these therapies may ease symptoms and slow or stop disease progression. Disease-modifying therapies typically fall into two categories: gene-silencing therapies and protein-stabilizing therapies.
Gene silencing therapies
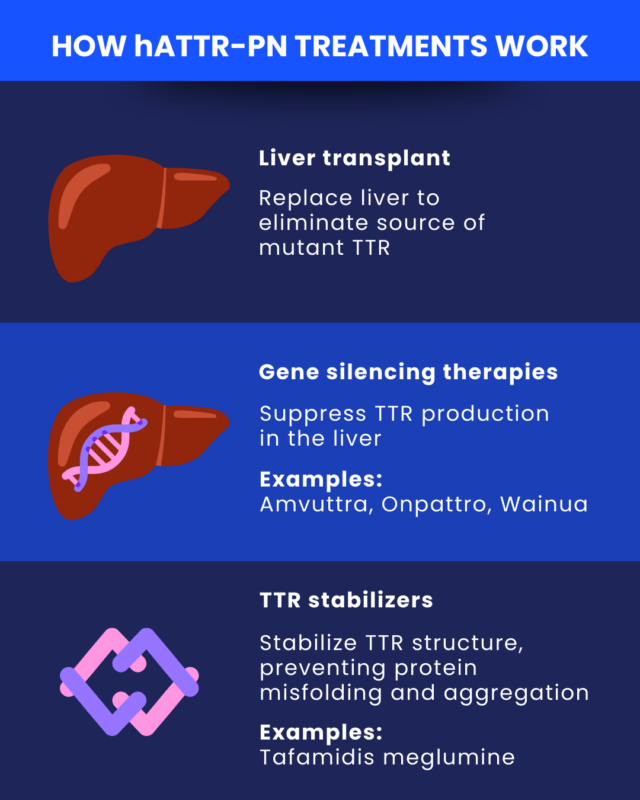
Gene silencing drugs for hATTR-PN suppress TTR production, effectively reducing the formation of toxic amyloid deposits. In the U.S., they are the recommended first-line treatment for hereditary TTR amyloidosis with polyneuropathy. Gene silencing therapies that are currently approved in the U.S. to halt or slow disease progression in hATTR-PN include:
These are administered via infusions into the bloodstream or under-the-skin injections. Potential side effects include infusion- or injection-related reactions and decreases in vitamin A levels.
TTR stabilizers
As the name suggests, TTR stabilizers work to stabilize the protein’s structure to prevent it from misfolding and forming toxic aggregates after it is produced. However, their long-term efficacy is still debated, and some pose risks of severe side effects. Some physicians use stabilizers for mild neuropathy symptoms, then switch to gene silencers if symptoms worsen.
No TTR stabilizers are currently approved in the U.S. for hATTR-PN. However, some therapies approved for other conditions that are found to have TTR-stabilizing effects have been used off-label to treat hATTR-PN, including diflunisal.
The TTR stabilizer tafamidis is approved in the U.S. to treat transthyretin amyloid cardiomyopathy (ATTR-CM), a form of TTR amyloidosis that affects the heart. It is marketed as Vyndamax. In Europe, tafamidis is approved as a therapy for ATTR-CM, and a different formulation, tafamidis meglumine, is approved for hATTR-PN. Both are sold as Vyndaqel.
Liver transplant
The liver is the primary site of TTR production. In people with hATTR-PN, liver cells produce an abnormal form of TTR due to mutations in the TTR gene. A liver transplant enables people with the disease to receive a healthy liver that produces the normal protein, thereby eliminating the source of mutant TTR.
Individuals with certain TTR mutations, particularly the Val30Met variant, may benefit from a liver transplant. To qualify for the procedure, they may need to meet specific criteria, such as being healthy enough to undergo transplant surgery.
However, the procedure is invasive, requires extensive recovery, and has mixed effects on long-term survival. Therefore, it is less frequently recommended in the era of new treatments for hATTR-PN.
Managing symptoms and complications
Additional interventions can help with symptom management in hATTR-PN, including:
- medications for pain associated with nerve damage
- anti-diarrheal medications for chronic diarrhea
- over-the-counter laxatives and stool softeners for chronic constipation
- medications to prevent nausea or vomiting
- medications to manage other potential symptoms of hATTR-PN, such as erectile dysfunction, low blood pressure when standing, delayed stomach emptying, and dry eye
- occupational therapy to develop strategies for completing daily activities
- physical therapy to improve mobility, strength, and balance
- mobility aids for motor symptoms
- wrist splints or surgery for carpal tunnel syndrome
- nutritional support to manage unintentional weight loss
In addition to polyneuropathy, people with hATTR-PN may have heart complications. Disease-modifying therapies can help address heart problems associated with TTR amyloidosis, although pacemakers and other interventions may also be considered if heart function is impaired.
Coordinated care and monitoring
After starting hATTR-PN medications, follow-up visits should be carried out at least every six months. With these regular appointments, a multidisciplinary hATTR-PN care team can evaluate disease progression and response to hATTR-PN treatments, which can take over a year to emerge fully. Neurologists, cardiologists, kidney specialists, and general practitioners may coordinate this care.
Genetic counseling may also be part of the diagnosis process. These specialists can help patients understand what to expect in terms of prognosis and make recommendations about family testing.
Daily care and support
In addition to medication and physical or occupational therapy, social support and mental healthcare are important for hATTR-PN daily care. Counseling may help patients and caregivers process emotions about the disease and its prognosis.
Resources from the Amyloidosis Research Consortium and the Amyloidosis Foundation can help patients and caregivers better understand amyloidosis and connect with the community. Patient support groups are also available throughout the U.S. and internationally.
For those interested in finding specialists or participating in hATTR-PN clinical trials, ARC’s My Amyloidosis Pathfinder tool may be a helpful resource.
Amyloidosis News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by